Partners
Planet Pharmacies UAE
MR Group KSA
AlDuaij Group Kuwait
- Published in YHB Group ltd
Branches
Young Health & Beauty Group Ltd. Taiwan 2007
YHB Group Ltd. Egypt 2013
YHB Group FZLLE UAE 2015
- Published in YHB Group ltd
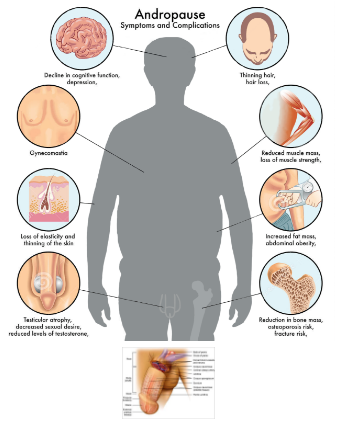
Andropause
Men in middle age facing major problems
-Hormonal disturbance
-Joints pain and arthritis
-Week erection and sexual activity
-Fatigue and powerless feelings and depression
- Published in Laennec
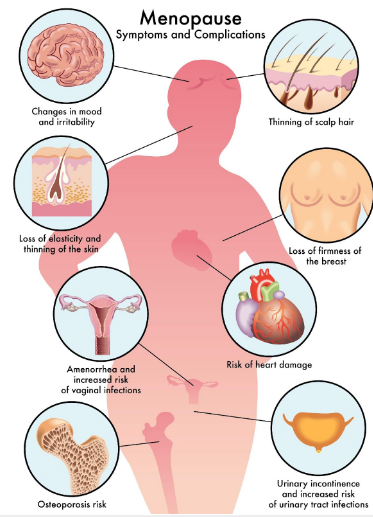
Post Menopause
Women in middle age facing major problems
-Headache, light sleep , anxiety, tension and mild depression
-By absence of estrogen calcium ions disappear from female bones become fractured so easy head of femur
-Due to lake of estrogen bad cholesterol LDL increase in blood stream causing atherosclerosis combined with active oxygen causing hardening in blood vessels
-At the beginning of sudden fall of estrogen causing vaginal inflammation (vaginitis) and loss of lining mucosal membrane of vagina which is a lubricant during sexual intercourse and act as anti-microbial function for vagina causing an atrophic vaginitis
-Menopausal disorders arise because of sudden fall in estrogen levels caused by reduction in the functions of ovaries which stimulates hypothalamus gland in brain (master of hormones in the body)
- Published in Laennec
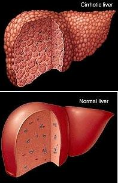
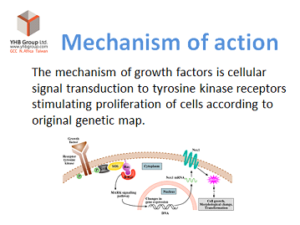
Hepatic Chronic Disease
Improvement of hepatic function in chronic hepatic disease (fatty liver, fibrosis and cirrhosis)
via regeneration of liver parenchymal cells and improvement of micro circulation
The bioactive ingredients of Laennec
-Hepatocyte Growth Factor (HGF): Promotes growth of liver parenchymal cells and various tissues
-Nerve Growth Factor (NGF): Promote growth of nerve cells (sensory and sympathetic ganglionic cells)
-Epidermal Growth Factor (EGF): Promote growth of skin, lungs, cornea and tracheal epithelial cells
-Fibroblast Growth Factor (FGF): Promote growth of human fibroblasts, glia cells and vascular endothelial cells
-Insulin-like Growth Factor (IGF): Promote growth of cartilage cells, and smooth muscle cells
-Growth Factors which Increase Immune Strength
-Colony-Stimulating Factor (CSF): Promote growth of stem cells such as immuno-competent cell granulocytes, and macrophages
-Interleukin-1 (IL-1): Promote production of immune-competent cell (T-cells, B-cells and NK-cells), thymus calls, and lymphokines
-Interleukin-2 (IL-2): Promotes growth of T-cells (helper T-cells, killer T-cells and suppressor T-cells)
-Interleukin-3 (IL-3): Promotes growth of hematopoietic cells, and mast cells
-Interleukin-4 (IL-4): Promotes growth of B-cells, and promotes division of antibody-producing cells
–Others
Nucleic Acids, Amino Acids, All kinds of Activate Peptides, Cytokines, Vitamins, Enzymes and Minerals
LAENNEC for Aesthetics and Anti Aging
LAENNEC stimulates normal growth, regenerates and repairs aged or injured muscle skin, collagen, cartilage, and nerve tissue which in turn shows improvement in epidermal elasticity, thickness, reduction of wrinkles, eye bags, dull skin tone, refining of facial pores, blotches, melesma, pigmentation, eczema, dermatitis, keloids, scars, etc. In our recent clinical trials, we have established a relationship between skin whitening and the use of LAENNEC.
LAENNEC helps to prevent and regulate aging and wear of different organs such as the brain, heart, lungs, liver, kidneys, and digestive system. It also promotes tissue respiration and Improvement in blood circulation, strengthening of immune system, concentration, eyesight and enhancement of stamina and energy.
Proven also to be effective for pre menopausal women by reduction in PMS symptoms and delaying menopause, LAENNEC increases sexual energy and appetite for both sexes.
LAENNEC helps in replenishing essential nutrients and quickens the healing of wounds, surgery and diabetic ulcers.
Stem cells are responsible for growth during childhood and for the repair and regeneration of human tissue throughout our lives. After the age of 25 the level of stem cells are only at 20 percent. As the levels of stem cells decline the damage that we call aging continues to accelerate. By increasing the levels of stem cells in our bodies, we can slow, or even reverse, many manifestations of aging. There is no other single therapy currently available that can have the impact on aging that Laennec Fresh Cell Therapy can have.
Laennec stem cell Growth factor therapy injection: It has been well believed that with people in their life span care most are those problems of health, juvenescence, and longevity. However, it is not until the revolution and advancement of 21st century biology technology, Human Placenta Extract can be conducted and utilized by human beings to bring new hopes in life.
Therefore, we want to introduce to you this new-age born premier product out of a prudent heart, namely the Laennec Human Placenta. Growth factor, which has been valued as the best product for human health.
Laennec Human Placenta injection adopt the quality placenta which had been passed detail selections of healthy expectant mothers. The quality is equal to those from France and Italy, but the quantity is 3 times more. Because of many constituents inside, the unit of ingredient indication is mg. Not only it has the attribute of being water-solubility, but also is a mature product that has been put in clinical practice for more than 60 years.
Benefits :
- Whitens skin
· Lightens facial pigmentations
· Refines facial pores and adds a glow to skin
· Improves skin elasticity and thickness
· Improves skin texture with a more evenly toned color
· Eliminates acne
· Revitalizes skin -
Reduces wrinkles
· Rapidly moisturizes to hydrate skin
· Reduces eye-bags
· Enhances the development of the breasts
· Firms sagging breasts
Aging-related Health Problems
- Lowers high blood pressure
· Regulates diabetes
· Treats hypertension
· Decreases serum concentrations of cholesterol and triglycerides
· Reduces gastric ulcers
· Reduces arthritis pain
· Improves blood circulation
Injuries and Tissue Damage
- Stimulates the regeneration of cells and tissues
· Speeds up the healing of wounds
· Speeds up recovery after surgery
Menopausal Syndrome
- Reduces the pain and changes associated with menopause
· Regulates menstrual periods
· Balances the hormones
Reproductive System
- Boosts up sex drive and potency with endurance and vitality
· Renews sexual satisfaction
· Treats impotence
· Increases sperm production
· Treats infertility and sterility (both male and female)
· Stimulates the production of milk in mothers
· Speeds up recovery after giving birth
- Prostatic hypertrophy
- Menopause and Andropause (male menopause)
- Ovarian Dysfunction
- Frigidity
- Oligogalactia
- Gestational toxicosis
General Health
- Boosts the immune system
· Vastly improves alertness and mentality
· Stabilizes weight
· Regulates and normalizes the autonomic nervous system
· Treats neurasthenia
· Eliminates asthma
· Eliminates constipation· Eliminates liver problems
· Treats gastrointestinal disorders
· Reduces the harmful effects of radiation
· Eliminates rheumatoid arthritis
· Improves physical fatigue
· Suppresses cough and mucus
· Promotes a deeper and more relaxing sleep
· Helps replenish required nutrients
· Improves general lack of vitality, concentration, eyesight and mental weakness
- Published in Laennec
Laennec P.O. Porcine
A capsule contains 350mg of 100% JBP placental extract (porcine), applying the same extraction method of Laennec injection, our ethical product extracted from human placenta, to the Japanese domestic porcine placenta. You will realice the benefit of pure placental extract without any additives or any other ingredients.
Ingredients per capsule
| Active ingredient | Amount | Function |
|---|---|---|
| Porcine Placenta Extract | 350 mg | Keeps body metabolism |
Presentation
100 Caps of 350 mg per box
Administration
3-6 Caps a day. Free from additives.
Recomended for:
• Maintain physiological functions and hormone balance
• Adjust autonomous nervous system
• Improves metabolism and blood circulation
• Promotes recovery from fatigue
• Enhances immunity and resistance
• Helps synthesis of collagen
• Suppresses the generation of melanin pigment
• Keeps and produces healthy skin
• Neutralizes active enzymes and prevent oxidation
• Supports liver function
- Published in Laennec
Curacen
JBP Curacen(R) is the ethical drug specialized for beauty/cosmetic purpose, because of its high penetration capability into the skin as it consists of the lower molecular weight substances from the human placenta.
- Published in Products
Laennec P.O.
Human placental supplement extracted by the same technology and safety protocol as Laennec injection. Designed the same efficacy and availability as two capsules equivalent to one ampoule of Laennec injection.
Anti-aging and dermatological effects
• Whitening and moisture retention
• Cell proliferation and reproduction
• Promoting self-adjustment/medication of immune and hormonal system
• Extracellular matrix generation
• Blood circulation promotion, etc…
Components in JBP Placental Extract
• Growth factors and cytokines: central element of placenta, demonstrating medicinal effects
• Aminoacid: essence of life
• Protein
• Carbohydrates: energy source
• Vitamin
• Nucleic acid and nucleobases
• Lipid and fatty acid
• Mucopolysaccharides: element to connect cells, works to suppress inflammation
• Mineral
• Enzyme
Presentation
100 caps of 350 mg/ box
Administration
2 ~6 Capsules per day (Additive Free)
- Published in Products
Laennec Injections
Laennec is the ethical drug manufactured with JBP’s unique technologies.
Laennec is the ethical drug manufactured with JBP’s unique technologies for effective extraction of variety of growth factors, cytokines, and other physiologically active substances from the human placenta. For instance, HGF (hepatocyte growth factor) promotes the proliferation of hepatic parenchymal cells for recovery of a damaged liver. Our product safety is ensured by the most rigid safety measures among existing scientific standards.
Warning: As the LAENNEC Injection is an ethical drug, JBP has never sold it on Internet.
| Ingredients | Content | Remarks | |
| Active ingredient | Water-soluble substance of a product of enzymatic human placenta | 112 mg | Ingredient extracted from human placenta |
| Inactive ingredients | Pepsin | Trace | Gastric mucosal extract (pig) |
| Lactose | 0.6 mg | – | |
| pH adjuster | q.s. | – |
Description
This product is a light yellow-brown or yellow-brown clear liquid with a distinctive odor. The pH level ranges from 5.5 to 6.5 and the osmotic pressure ratio (to physiological saline) is approximately one.
Indications
Improvement of hepatic function in chronic hepatic disease.
Dosage and administration
The normal adult dose is a 2 ml subcutaneous or intramuscular injection once daily. According to symptoms, the dose can be increased to 2 or 3 times daily.
Packaging
2 ml 50 ampoules
Cautions for use**
1. Careful Administration
LAENNEC should be administrated with care in patients predisposed to allergies.
2. Important Basic Cautions
This product is manufactured from the extract of human placenta delivered full-term in Japan. In order to screen each donor, a complete medical history, interviewing such as a history of travel and serologic testing for viruses, bacteria and infection are performed, after nucleic-acid testing (NAT) to meet with requirements for HBV-DNA, HCV-RNA and HIV-1-RNA is carried out. In addition, it has been confirmed that high-pressure steam sterilization for 20 minutes at 121 °C during the manufacturing process is effective in inactivating various viruses such as HIV etc. Furthermore, although in the product test, the nucleic-acid test meets with requirements for HBV-DNA, HCV-RNA, HIV-1-RNA, HTLV-DNA, and parvovirus B19-DNA, patients should be made aware of the following points during administration: To date, the transmission of infection, such as variant Creutzfeldt-Jakob disease (vCJD), by administration of this product in Japan or other countries has not been reported. However, although safety measures are taken to prevent infection during the manufacturing process, it is theoretically impossible to fully eliminate the risks of infection transmission originating in a human placenta used as raw material. While safety measures during the manufacturing process to prevent infection, as well as confirmation of the necessity of treatment for the disease before administration are carried out, doctors should explain to patients and try to have them understand that when human placenta is used as the raw material of a product, the risk of infection cannot be fully ruled out.
3. Drug Interactions
When this product is directly mixed with a strong base preparation of pH 8.5 or more, attenuation of pharmacological activity has been reported.
No coadministration with this product resulting in the enhancement or attenuation of the pharmacological effect of this product or concomitant drugs, appearance of adverse reactions, or aggravation of disease has been reported.
Adverse reactions or patients who were suspected to have suffered adverse reactions to this product were reported in a total of 10 (3.7 %) of the 273 patients selected for safety evaluation in the clinical study performed during implementation of reevaluation of the drug efficacy. The most frequently observed adverse reactions were injection site pain in 7 patients (2.6 %), hypersensitivity (such as rash, fever, and itching) in 1 patient (0.4 %), injection site indurations in 1 patient (0.4 %), and gynaecomastia in 1 patient (0.4 %). The causal relationship between gynaecomastia and this product is unknown.
No abnormal changes in laboratory values were observed.1)
• Clinically significant adverse reactions
**Shock (incidence unknown):
Since this product is a protein/amino acid preparation derived from human tissue, this product may cause a shock. If any signs of abnormality are observed, the drug should be discontinued immediately, appropriate measures should be taken and the condition should be monitored fully.
• Other adverse reactions (in descending order of occurrence)
| Injection site pain | 2.56% |
| Sensitivity (rash, fever, itching etc.) | 0.37% |
| Injection site indurations | 0.37% |
| Gynaecomastia | 0.37% |
4. Use in the Elderly
From clinical data and present use, no particular caution is needed in the administration of this product to the elderly. However, since elderly patients often have reduced physiological function, it should be administrated with care.
5. Use during Pregnancy. Delivery or Lactation
In reproductive development toxicity experiments on animals, including teratogenicity, this product can be considered not to have such toxicity.2)
6. Pediatric use
The safety of this product in premature infants, newborns, infants, toddlers or children has not been established due to insufficient clinical data.
7. Overdosage
Overdosage of this product and the resulting efficacy or safety have not been established (Due to insufficient clinical data).
8. Precautions for Use
Injection site:
In order to avoid any effect on tissue or nerves, the product should be injected subcutaneously or intramuscularly taking the following precautions:
a) For the injection site, to avoid nerve pathways, it should be administrated with care.
b) In the case of repeated injections, avoid injecting into the same site by alternating on the left and right side etc.
c) If intense pain or regurgitation of blood is observed, the needle should be removed immediately, and injected into a different site.
Opening the ampoule:
When opening the ampoule, it is preferable to wipe the part to be cut with an ethanol sponge before opening it.
Pharmacokinetics
The bioactive ingredients of LAENNEC are extracted from human placenta, and the primary pharmacological effects of this product can not necessarily be attributed to a single substance or ingredient. Therefore, evaluation of the pharmacokinetics (absorption, distribution, metabolism, and excretion) of this product has not been established.
Clinical studies
1. Double blind comparison to chronic hepatitis and hepatic cirrhosis1)
In a double-blind crossover study of 124 patients in Japan on the effects of this product on chronic hepatitis and hepatic cirrhosis, administration of this product improved serum transaminase (GOT, GPT) levels significantly (see figures below).
Efficacy to GOT

Efficacy to GPT


Summary of drug-efficacy
[(A) Items having a tendence to decrease or increase significantly by Laennec]
Data on pharmacological reevaluation
Summary of drug-efficacy
[(B) Items having a tendence to decrease or increase significantly by Placebo]

2. Action on HCV-RNA levels3)
Twenty subjects of two-agent combination therapy only with Stronger Neo-Minophagen C and Acelart were performed in the past and negativation neither of C100-3 antibody nor HCV-RNA was admitted in them. In three-agent combination therapy with Laennec added, two negative cases of serum HCV-RNA levels were admitted.
Pharmacology
1. Stimulatory effects of hepatic reproduction4), 5)
After 70 % of livers in healthy rats was resected, time comparisons of liver weight to the control groups resulted in significant stimulation of hepatic reproduction in the Laennec groups.
2. Stimulatory effects of cellular DNA synthesis4)
Compared to control groups, in vivo experimental system using primary culture rat hepatocytes, the DNA-synthesis accelerator activity was observed in 2 μL/ml-administrated groups. That accelerator activity was equivalent to 10 pM of rHGF. The liver tissue in vivo experimental system using rats with acute hepatitis by ANIT was stained and the result of evaluation of the rate of hepatocyte nucleus in DNA synthesis period showed significant accelerator of DNA synthesis compared to the control groups.
3. Inhibitory effects of experimental liver injury4), 6)
Compared to control groups, in vivo experimental system using rats with acute hepatitis by ANIT, the liver cytosolic enzymes in serum (GPT, ALP, LAP and g-GTP) and the bilirubin levels significantly degreased in the Laennec groups.
In addition, compared to control groups, in vivo experimental system using rats with acute hepatitis and chronic hepatitis by CCI4, the liver cytosolic enzymes in serum (GPT, GOT) significantly degreased and histopathologically improved liver disorder in the Laennec groups.
4. Anti-fatty liver action7)
A 5-day subcutaneous injection of 1.2 ml/kg of this product in rats suffering from acute hepatic disorder with CCI4 resulted in a significant decrease in hepatic weight to body weight as compared with the control groups. Furthermore, total hepatic lipids and total hepatic cholesterol decreased significantly in the 3.6 ml/kg dose groups, and hepatohistological findings to support such results were seen and the body weight was realized.
After pretreatment of acute hepatic disorder with CCI4, there were no significant differences with regards to biochemical findings between the control groups and when 5-day repeated, subcutaneous administration of 1.2 ml/kg of this product was performed. However, in the 3.6-ml/kg dose groups, maintenance of lobular structure was better than the control groups with regards to hepatohistological findings.
5. Absorption accelerated activity on interstitial connective tissue8)
This product exhibited inhibitory action of fibroplasias in rat liver due to 12-week repeated administration of CCI4, and it was confirmed histologically that once multiplied, interstitial connective tissues were absorbed.
Physicochemistry
This product is human-placental extract, and contains various biological active substances. However, the active ingredients of this agent cannot be specified as a single substance or several sorts of substances.
Precautions for handling*
This product corresponds to a specialized biological product. Therefore, when this product is administrated, the product name, manufacturing No., date of administration (or prescription), name and address of patients should be reported and stored for at least 20 years in accordance with the order issued by the Japanese Ministry of Health, Labour and Welfare. This product should be used with attention to the point that the indication of this product is “improvement of hepatic function in chronic liver disease”.
References
1) Ueda H. et al.: Kanzo (Liver), vol.15, 162, 1974
2) Taniguchi H. et al.: data on file in Japan Bio Products Co. Ltd., 1985-1991
3) Takami T.: Kiso to Rinsho, vol.30, 3549, 1996
4) Liu K. et al.: Yakuri to Rinsho, vol.5, 2187, 1995
5) Sakamoto K. et al.: Journal of Tokyo Medical University, vol.33, 271, 1975
6) Nakayama S. et al.: Folia Pharmacologica Japonica, vol.94, 289, 1989
7) Sakamoto K. et al.: Journal of Tokyo Medical University, vol.31, 829, 1973
- Published in Products
liver
-Pure human placental extract free of blood , hormones and steroids.
-Placenta donors selected with high selectivity profile to be sure they are free of diseases to the 4th generation depend on Japan’s governmental data.
-Manufactured by a unique technique to ensure product free of any biological contamination.
-Laennec is an ethical product for all types and stages of liver cirrhosis in japan since 1974.
-Laennec is registered in Taiwan,Korea,Switzerland ,china and recently Gulf countries,Saudia Arabia and Egypt as a liver cirrhosis treatment.
medicinal functions
of LAENNEC:
-Improve immune system strength .
-improve microcirculation and blood production.
-hormonal regulatory function.
-Nervous system regulatory function.
-Tissue repair function.
-Tissue regeneration function .
-Basal metabolism function: energies the metabolism and activating organs.
– Anti oxidant function.
-Anti inflammation function.
-Anti mutagenic function.
-Fatigue recovery function .
Indications:
Improvement of hepatic function in chronic hepatic diseases.
Hepatic regeneration promotion action in liver cirrhosis cases.
chronic pancreatitis, diabetes, atherosclerosis, gastric and duodenal ulcers.
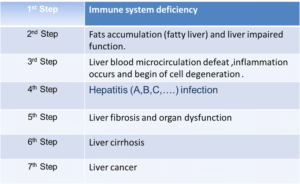
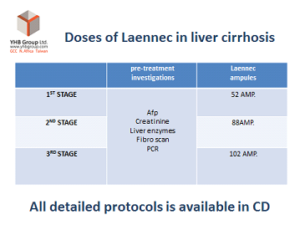
STEPS OF LIVER DAMAGE:
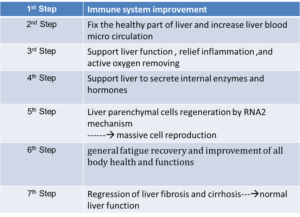
Steps of liver recovery by Laennec:
-Results depend on individual response variation.
– Change bad habits such as (Alcoholics, Smoke, Late Sleeping) doing exercises and follow diet regime .
– Patient must follow the daily/weekly doses and continue the course.
Safety profile:
Our product safety is ensured by the most rigid safety measures among existing scientific standards world wide(Switzerland and Italy).
Rare reported side effects as in following Japanese MOH report in the period of Jan 1996 – July 2015 on 5,600,000 patients.
Contraindications:
During Breast Feeding.
During Chemo-therapy.
Infant less than 10 years old.
During Pregnancy.
Hyper sensitivity.
- Published in Laennec
- 1
- 2